Considerations
Nitrogen Transformations in Aquaponic Systems
Nitrogen cycle
Nitrogen is a vital element for all living organisms because it is an essential component of deoxyribonucleic acid (DNA), ribonucleic acid (RNA) and protein. Arguably, the nitrogen cycle is the most important process in aquaponics. Fig. 1 shows the schematic diagram of nitrogen cycle in aquaponics.
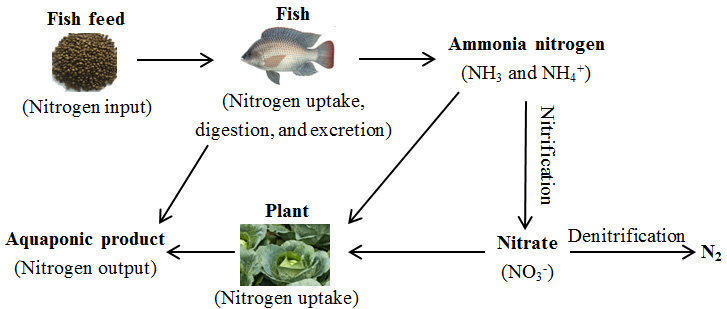
Fig. 1 Nitrogen cycle in aquaponics
The nitrogen cycle starts when fish feed is first added to the aquaponic system. Protein-rich fish feed is the major source of nitrogen in aquaponic systems. On average, only about 25-30% of nitrogen in the fish feed can be converted to fish biomass, while most of the nitrogen digested by fish is excreted into the aqueous phase as ammonia, a byproduct of protein metabolism. In water, ammonia exists in two forms: un-ionized ammonia (NH3), and ionized ammonium (NH4+). The sum of both forms is referred to as “total ammonia nitrogen (TAN).” There exists an equilibrium between NH3 and NH4+. The relative distribution of the two forms present in the aqueous phase is mainly affected by pH. In general, less than 10% of TAN is in the toxic form (i.e., NH3) when the pH is less than 8.0. However, this distribution increases dramatically as pH increases. The equilibrium between NH3 and NH4+ is also affected by temperature. At any given pH, more NH3 is present in warm water than in cold water.
NH3 + H2O ↔ NH4+ + OH-
NH3 is toxic to fish if allowed to accumulate in aquaculture systems. So it has to be diluted to a non-toxic level or converted into a less toxic form of nitrogen, e.g., nitrate (NO3-). When the ammonia levels in the fish tank reach a certain level, microbes begin to oxidize NH3/NH4+ to nitrate (NO3-). The process is known as nitrification.

Fig. 2 Molecular structure of NH3 and NH4+
Nitrification occurs under aerobic environments and is carried by two groups of autotrophic bacteria in two steps. NH4+ is first oxidized to nitrite (NO2-) by ammonia-oxidizing bacteria (AOB) (e.g., Nitrosomonas europaea or Nitrosococus oceani) and then further converted to nitrate by nitrite-oxidizing bacteria (NOB) (e.g., Nitrobacter winogradsky). The following equations describe the nitrification process.

It should be noted that the first step of nitrification could produce acid, which results in a decrease in pH. Since nitrification will be inhibited under low pH, base is needed to buffer the acid produced during nitrification.
In aquaponics there may exist non-turbulent areas where denitrification could occur. Denitrification is performed primarily by heterotrophic bacteria, under anoxic conditions, which is comparable to anaerobic conditions except for the presence of nitrate and/or nitrite. During denitrification, nitrate is reduced to nitrite and then to nitrous oxide, which is finally reduced to nitrogen gas.
NO3− → NO2− → NO + N2O → N2 (g)
The complete denitrification process can be expressed as the following equation. For each 1 mg/L of nitrate reduced to nitrogen gas, you will recover 3.5 mg/L of alkalinity.

When water flows through the hydroponic component of the system, NO3- and NH4+ can be taken up by plants. However, NO3- is often the preferred form of nitrogen. After the nitrogen is consumed, the purified water is then returned to the aquaculture component.
Factors influence nitrification
The main factors that affect nitrification rates are temperature, pH, and dissolved oxygen (DO) concentration.
- Temperature - The temperature for optimum growth of nitrifying bacteria is between 77-86 ºF (25-30ºC). Growth rate decreases by 50% when the temperature is decreased from the optimal to 64ºF (18ºC) and nitrifying activities cease when the temperature falls below 32ºF (0ºC) or above 120ºF (49ºC). It should be noted that Nitrobacter is less tolerant of low temperatures than Nitrosomonas. In cold water systems, care must be taken to monitor the accumulation of nitrites.
- pH - The optimum pH range for Nitrosomonas and Nitrobacter is between 7.8-8.0 and 7.3-7.5, respectively. Nitrosomonas growth is inhibited at pH below 6.5. All nitrifying activity is inhibited if pH drops to 6.0 or less. However, the optimum pH for plant nutrient availability in hydroponics is between 5.5-6.5. It is recommended that the pH of aquaponic systems be maintained at around 7.0.
- DO - Nitrifying bacteria are aerobic and their activity is affected by the DO in the aqueous phase. Maximum nitrification rates exist if DO levels exceed 80% of saturation. Nitrification ceases when DO concentration drops to 2.0 mg/L or less. The activity of Nitrobacter is more strongly affected by low DO than that of Nitrosomonas.
In addition, nitrifying activity can be enhanced by adding bacteria from an existing colony, including commercial nitrifying microbes, media from an existing aquaponics system, etc.
Nitrous oxide (N2O) emission from aquaponics
During nitrogen transformations, N2O can be produced through nitrification and denitrification. N2O is a potent greenhouse gas (GHG) with the global warming potential 296 times that of CO2. Although atmospheric N2O accounts for only 6% of the greenhouse effect, its high increase since 1990s (currently 0.25−0.30% per year) has aroused great concern, especially with respect to its sources and sinks.
Aquaponics might be an important source of N2O. The possible pathways of N2O emission from aquaponic are illustrated in the following figure. In our study, a Clark type electrode N2O sensor (Unisense, Aarhus N, Denmark) was used to determine the N2O emissions from aquaponics. The N2O conversion ratio varies from 1.5 to 8.0%, depending on temperature, DO, ammonium and nitrate concentrations.
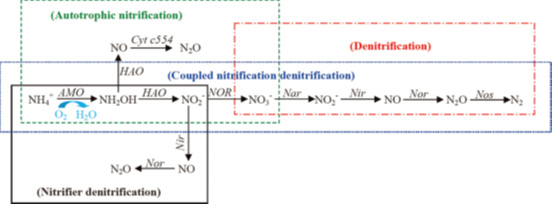
Fig.3 Possible pathways of N2O emission during nitrogen transformations