STAINING TECHNIQUES
(Read about staining techniques in Tortora et. al., Chapter 3 and about gram positive and negative cells and their cell walls in Chapter 4, especially Table 4.1. Also look up all of these stains in your Leboffe and Pierce book.)
THE GRAM STAIN
This is a DIFFERENTIAL STAIN. It requires a PRIMARY STAIN and a COUNTERSTAIN. It divides most of the EUBACTERIA into two large groups: GRAM POSITIVE bacteria and GRAM NEGATIVE bacteria.
The basic procedure goes like this:
1. Take a heat fixed bacterial smear.2. Flood the smear with CRYSTAL VIOLET, 1 minute, then wash with water. [PRIMARY STAIN]
3. Flood the smear with IODINE, 1 minute, then wash with water. [MORDANT]
4. Flood the smear with ETHANOL-ACETONE, quickly, then wash with water. [DECOLORIZE]
5. Flood the smear with SAFRANIN, 1 minute, then wash with water. [COUNTERSTAIN]
6. Blot the smear, air dry and observe.
- EXPERIMENT:
- 1.)Perform the gram stain on smears made form the following organisms:
- Staphylococcus aureus
- Escherichia coli
- Corynebacterium minutissimum
2. ) Use a sterile applicator stick to obtain a sample of the "crud" on your teeth at the gum line.
Mix the sample with a loopful of water on a microscope slide and allow the specimen to air dry.
Heat fix and perform the gram stain on this sample.3.) Observe all of your specimens under oil immersion and record cell morphology and arrangement and gram reaction.
GRAM (+) organisms hold onto the crystal violet-iodine complex more tightly than the GRAM (-) bacteria do. However this is not an absolute phenomenon! Sometimes gram (+) cultures will appear gram (-) or GRAM VARIABLE (a mixture of gram (+) and gram (-) cells). What are some of the reasons for this?
2. Too harsh heat fixation
3. Too thick a smear
4. Improper washing between steps
5. Too old a culture
6. Impure or mixed culture
It is believed that the gram staining characteristics of an organism is a function of its cell wall. Therefore any time the cell wall is damaged the gram stain characteristics of gram positive cells will change. On the other hand, gram (-) cells never become gram (+) if the stain procedure is done correctly.
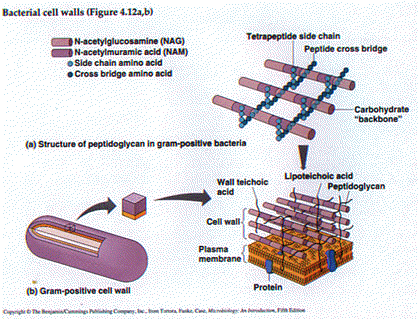
There are fundamental biological differences between gram (+) and gram (-) cells. These are summarized in Table 4.1(Tortora et. al.) and I'd like to stress the following points:
1. Gram (+) cells have thicker cell walls - more peptidoglycan and teichoic acid.2. Gram (-) cells have Lipopolysaccharide (Endotoxin) in the outer membrane of their cell walls.
3. Gram (+) cells are generally more sensitive to those antibiotics which interfere with cell wall biochemistry like penicillin, vancomycin and cephalosporin.
4. Gram (+) cells are more sensitive to lysozyme - a peptidoglycan digesting enzyme.


The gram morphology of some common bacteria:
Gram (+) = Staphylococcus, Streptococcus, Bacillus, ClostridiumGram (-) = the coliforms: (Escherichia, Klebsiella, Serratia), the enteric pathogens: Salmonella, Shigella, Campylobacter
THE CAPSULE STAIN - The Gin Stain
Most bacteria have some kind of CAPSULE. This viscous surface layer is also known as the SLIME LAYER, the GLYCOCALYX or the EXTRACELLULAR POLYMERIC SUBSTANCE (EPS). Most bacterial capsules are composed of polysaccharide however some genera produce polypeptide capsules. Capsular material is very moist (slimy) and any heating will cause it to shrink - it is for this reason that we will not heat fix the slide before staining. Also, heating may cause the bacterial cell to shrink resulting in a clear zone around the cell - which may cause cells which don't have capsules to appear as if they do.
The polymers which make up the capsule tend to be uncharged and as such they are not easily stained. For this reason we use a NEGATIVE STAIN to visualize them. That is, we use a stain which stains the background against which the uncolored capsule can be seen. Our procedure, the Gin's Method, uses india ink to color the background and crystal violet to stain the bacterial cell "body"
This structure helps the bacterial cell to ATTACH TO SURFACES and
to AVOID BEING PHAGOCYTOSED. For instance, the oral
streptococci produce a glucan based EPS which helps them to attach to
the teeth. When this material begins to accumulate on the teeth
it is referred to as dental plaque. As a general
phenomenon, organisms with capsules tend to be more virulent
presumably because of their resistance to phagocytosis and killing.
Streptococcus pneumoniae exists in a smooth form (encapsulated) and a
rough form (non-encapsulated). Only the smooth form is lethal for
mice.
- EXPERIMENT
- a. Use a loop to mix a drop of water, a drop of india ink and a small amount of Klebsiella pneumoniae together at the end of a slide.
b. Use another slide to spread the smear like a blood smear. (As the instructor will demonstrate.) Allow the smear to air dry. DON'T HEAT FIX!
c. Flood the smear with crystal violet, 1 minute. Wash with water, blot, dry, observe. Compare your observations with the illustrations on page 30 of Leboffe and Pierce.
ENDOSPORES - The Schaeffer - Fulton Stain
These are very resistant structures made by only a few genera of bacteria. The two genera which we will study are:
Bacillus is a common aerobic genus whose species can form endospores. Anthrax and Bacillus cereus food poisoning are two diseases caused by members of this genus.
Spores are extremely resistant structures, difficult to destroy
with heat or other physical and chemical disinfecting agents.
Endospore destruction is the standard for testing the operation of an
autoclave.
- EXPERIMENT
- Prepare a smear of Bacillus megaterium, allow the smear to dry and then heat-fix.
- Place the slide on the staining rack in the sink and flood the smear with malachite green stain.
- Heat the stain to steaming by passing a lit bunsen burner over the smear. Don't overheat the stain! Once the steaming stops, pass the bunsen burner over the slide again. As the stain evaporates add more stain. Continue this procedure for 5-10 minutes.
- (Safety Note: Please remember that the acetone-alcohol decolorizer from the gram stain experiment is extremely flammable. Do not perform this flaming step while people are gram staining!)
- Wash the smear gently and thoroughly with running water.
- Counterstain with aqueous safranin for 1 minute.
- Wash the slide with water, blot gently and allow the smear to air dry.
- Observe under oil immersion and compare what you see with the illustrations in Leboffe and Pierce.