
Soil Acidification by weathering
Department of Agronomy and Soil Science, College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa
____________________________________________________ Item pH ______________________________________________ Most acid soils 4.0-6.0 Lemon juice 2.2-2.4 Orange juice 3.4-4.0 Vinegar 4.0-4.5 Acid rain 3.0-5.0 Clean rain water 5.5-5.7 Fresh milk 6.3-6.6 Blood plasma 7.0-7.2 Mild soap solution 8.5-10.0 ______________________________________________________

 Aluminum is more soluble under acidic conditions; and high Al levels are toxic
to plants. Aluminum toxicity usually damages the root system first.
Aluminum-affected roots tend to be shortened and swollen, having a
stubby appearance.
Aluminum is more soluble under acidic conditions; and high Al levels are toxic
to plants. Aluminum toxicity usually damages the root system first.
Aluminum-affected roots tend to be shortened and swollen, having a
stubby appearance. Unlike Al, Mn toxicity first shows up in plant tops. The symptoms vary
among plant species, but often are specific for a given species. For example,
stunted, crinkled, and chlorotic leaves are the Mn toxicity symptoms in soybeans.
Unlike Al, Mn toxicity first shows up in plant tops. The symptoms vary
among plant species, but often are specific for a given species. For example,
stunted, crinkled, and chlorotic leaves are the Mn toxicity symptoms in soybeans.
 Unlike acid soils of the US mainland, acid soils in Hawaii are often Ca-deficient rather than Al-toxic. This problem is particularly severe in Kauai, the oldest island in the State. Since Ca is fairly immobile inside the plant, its deficiency symptoms
first appear at the growing points. In corn and taro, Ca-deficient plants are stunted; young
leaves are unable to fully unfurl then the leaf tips or margins soon die.
Unlike acid soils of the US mainland, acid soils in Hawaii are often Ca-deficient rather than Al-toxic. This problem is particularly severe in Kauai, the oldest island in the State. Since Ca is fairly immobile inside the plant, its deficiency symptoms
first appear at the growing points. In corn and taro, Ca-deficient plants are stunted; young
leaves are unable to fully unfurl then the leaf tips or margins soon die.
 Phosphorus can react strongly with Fe and Al components of acid tropical
soils, thereby becoming unavailable for plant uptake. Older leaves in P-deficient
plants are often purple because of the accumulation of anthocyanins (purple pigments).
Phosphorus can react strongly with Fe and Al components of acid tropical
soils, thereby becoming unavailable for plant uptake. Older leaves in P-deficient
plants are often purple because of the accumulation of anthocyanins (purple pigments).
Although planting acid-tolerant crops is a reasonable option for dealing with acid soils, liming is traditionally used to correct soil acidity and to improve soil productivity.Thus liming eliminates toxic Al3+ and H+ through the reactions with OH-. Excess OH- from lime will raise the soil pH, which is the most recognizable effect of liming. Another added benefit of liming is the supply of Ca2+ and Mg2+ if dolomite [Ca,Mg(CO3)2] is used.When lime (i.e., CaCO3) is added to a moist soil, the following reactions will occur: (1) Lime is dissolved slowly by moisture in the soil to produce Ca2+ and OH- CaCO3 + H2O (in soil) ==> Ca2+ + 2OH- + CO2 (gas) (2) Newly produced Ca2+ will exchange with Al3+ and H+ on the surface of acid soils 2Ca2+ + soil-Al ===> soil-Ca + Al3+ + soil-H soil-Ca + H+ (3) Lime-produced OH- will react with Al3+ to form Al(OH)3 solid and with H+ to form water. Al3+ + 3OH- ===> Al(OH)3 (solid) H+ + OH- ===> H2O
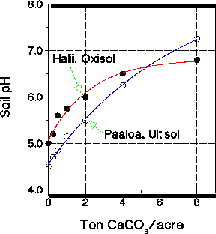
Because soils differ widely in mineralogy, organic matter and clay content, they require different amounts of lime to raise soil pH to a given value. Thus lime requirement curves should be constructed for individual soils to be used in lime estimation. Figure 1 shows an example of such curves.
____________________________________________________ Crop pH -------------------------------------------------- Alfalfa 6.5-7.5 Avocado 6.0-6.5 Azalea 4.5-5.0 Camelia 4.5-5.5 Ginger 6.0-7.0 Macadamia 5.0-6.5 Pineapple 4.7-5.7 Sugarcane 6.0-7.0 Taro 5.5-6.5 _____________________________________________________